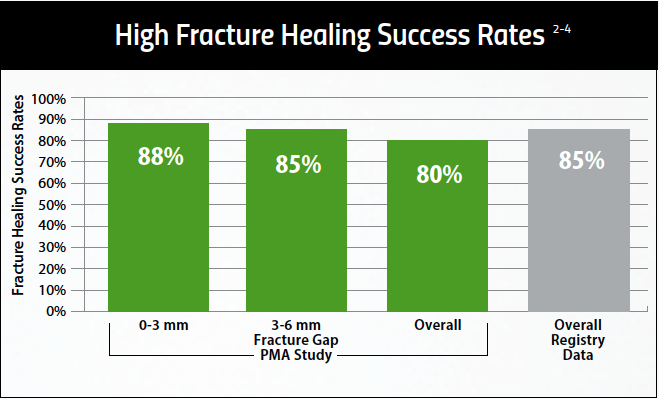
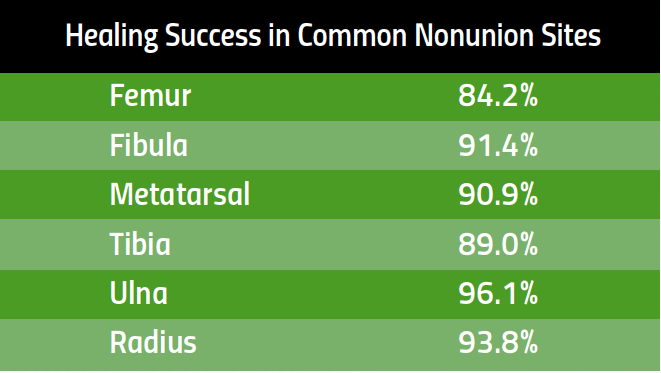
Healing Success in Common Nonunion Fractures
PhysioStim™ devices provide a safe and effective non-surgical treatment to improve nonunion fracture healing. These devices use a pulsed electromagnetic field (PEMF) signal to induce a low-level electrical field at the fracture site which stimulates bone healing. 2,3
Proven Effective Therapy
Anatomically Designed Models

- 360 degrees of PEMF treatment around the fracture site that evenly penetrates across tissue, bone and fixation1,7
- Single-piece, cordless design that allows for ease of placement and patient mobility
Commitment to Outcomes
PhysioStim devices are accompanied by the STIM onTrack™ mobile app, which provides patients with a treatment calendar, therapy reminder, outcome measurement tools and additional educational resources.
STIM onTrack mobile app combined with the Orthofix DIRECT™ Physician Portal enables physicians to remotely:
- View patient adherence to prescribed treatments
- View patient reported outcome measures (PROM) questionnaire responses
- Manage new and submitted patient prescriptions
Brief Prescribing Information:
The PhysioStim™ device is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Use of this device is contraindicated where the individual has synovial pseudarthrosis. Demand type pacemaker operation may be adversely affected by exposure to pulsed electromagnetic fields. The safety and effectiveness of this device has not been established for individuals lacking skeletal maturity or individuals with a nonunion secondary to, or in connection with, a pathological condition. The safety of this device for use on patients who are pregnant or nursing has not been established. Rare instances of reversible minor discomfort have been reported.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Services at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
References: 1. Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic 2. PMA P850007. February 1986 3. Garland DE, Moses B, Salver W. Fracture healing: Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 1991;22(3):295-302 4. Orthofix patient registry. PMA P850007/S20. Data on file. 5. iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP19_RPT), iData Research Inc (www.idataresearch.net) 2017 6. iData Research Inc., U.S. Market for Orthopedic Trauma Devices (iDATA_USTRA19_RMS), iData Research Inc (www.idataresearch.net) 2019 7. Navarro, M., Michiardi, A., Castano, O., & Planell, J.. (2008). Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5(27), 1137-1158