Increase Success Rates in Spinal Fusion
The SpinalStim™ device provides a safe and effective non-surgical treatment to improve fusion healing. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing.2-4
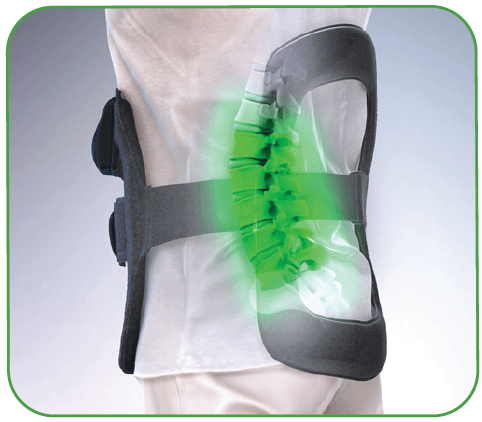
The only bone growth stimulation therapy device approved by the FDA as both a lumbar spinal fusion adjunct and as a non-surgical treatment for spinal pseudarthrosis.2-4
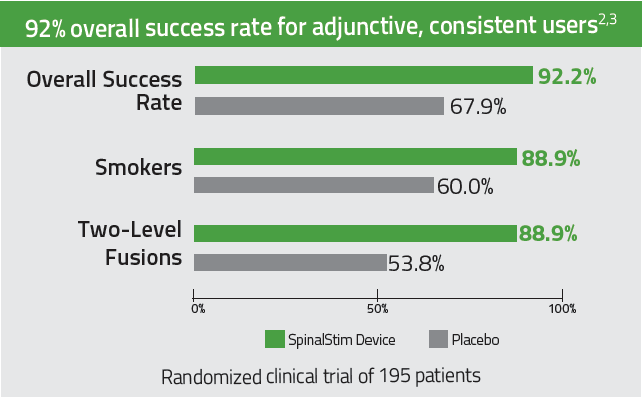
92% overall clinical success rate for adjunctive, consistent users. 2-3
Improves success rates in spinal fusions by 36% 2,3
- 360 degrees of pulsed electromagnetic field (PEMF) treatment around the fusion site that evenly penetrates across tissue, bone and fixation5,6
- PEMF coverage up to 5 vertebral levels5
- NASS coverage recommendations support the use of PEMF stimulation as an adjunct to spinal fusion surgery in high-risk patients7
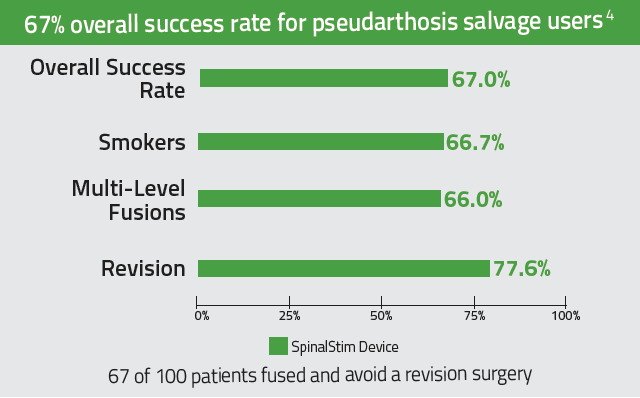
- Adjunctive PEMF treatment can be recommended for patients who are at high risk for pseudarthrosis8
Brief Prescribing Information:
The SpinalStim™ device is indicated as a spinal fusion adjunct to increase the probability of fusion success and as a nonoperative treatment of salvage of failed spinal fusion, where a minimum of nine months has elapsed since the last surgery. Cardiac pacemakers may be adversely affected by exposure to pulsed electromagnetic fields. Use of this device is contraindicated where the individual has an implanted cardiac pacemaker.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Services at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
References: 1. iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP19_RPT), iData Research Inc (www.idataresearch.net) 2017. 2. PMA P850007/S6. February 1990. 3. Mooney V. Pulsed electromagnetic fields: an adjunct to interbody spinal fusion surgery in the high risk patient. Surg Technol Int 1993, 2:405-410. 4. Simmons JW Jr, Mooney V, Thacker I. Pseudarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electro-magnetic fields. Am J Orthop. 2004;33(1):27-30. 5. Zborowski M, Androjna C, Waldorff EI, Midura RJ. Comparison of therapeutic magnetic stimulation with electric stimulation of spinal column vertebrae. IEEE Transactions on Magnetics, Vol. 51, No. 12, December 2105, 5001009. Erratum in IEEE Transactions on Magnetics, Vol. 53, No. 2, February 2017, 9700101. 6. Navarro, M., Michiardi, A., Castano, O., & Planell, J.. (2008). Biomaterials in ortho-paedics. Journal of the Royal Society Interface, 5(27), 1137-1158. 7. Spine.org. 8. Ethan Cottrill, MS, Zach Pennington, BS, A. Karim Ahmed, BS, Daniel Lubelski, MD, Matthew L. Goodwin, MD, PhD, Alexander Perdomo-Pantoja, MD, Erick M. Westbroek, MD, Nicholas Theodore, MD, Timothy Witham, MD, and Daniel Sciubba, MD Department of Neurosurgery, The Johns Hopkins School of Medicine, Baltimore, Maryland The effect of electrical stimulation therapies on spinal fusion: a cross-disciplinary systematic review and meta-analysis of the preclinical and clinical data SOURCE: J Neurosurg Spine, October 8, 2019.