- Drug-free pain relief
- Wearable design for therapy on the go
- Noninvasive and easy-to-use
- Helps return your patient to an active lifestyle
-
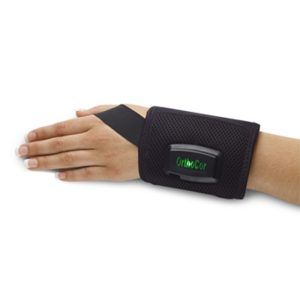 The OrthoCor Active System is indicated for adjunctive use in the palliative treatment of post-operative pain and edema in superficial soft tissue. The Active System temporarily relieves minor muscle and joint aches and pain associated with overexertion, strains, sprains, and arthritis. OrthoCor is the only wearable device on the market with patented Pulsed Electromagnetic Field (PEMF) therapy, one of the most advanced restorative therapies available today. PEMF works at the source of the injuries, and helps accelerate your body's natural anti-inflammatory and recovery responses. Treatment sites include back, knee, shoulder, ankle, wrist and elbow.
The OrthoCor Active System is indicated for adjunctive use in the palliative treatment of post-operative pain and edema in superficial soft tissue. The Active System temporarily relieves minor muscle and joint aches and pain associated with overexertion, strains, sprains, and arthritis. OrthoCor is the only wearable device on the market with patented Pulsed Electromagnetic Field (PEMF) therapy, one of the most advanced restorative therapies available today. PEMF works at the source of the injuries, and helps accelerate your body's natural anti-inflammatory and recovery responses. Treatment sites include back, knee, shoulder, ankle, wrist and elbow. -
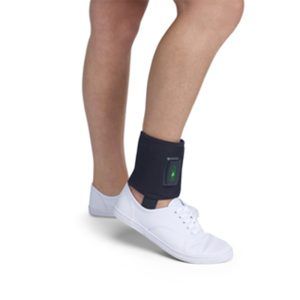 The OrthoCor Active System is indicated for adjunctive use in the palliative treatment of post-operative pain and edema in superficial soft tissue. The Active System temporarily relieves minor muscle and joint aches and pain associated with overexertion, strains, sprains, and arthritis. OrthoCor is the only wearable device on the market with patented Pulsed Electromagnetic Field (PEMF) therapy, one of the most advanced restorative therapies available today. PEMF works at the source of the injuries, and helps accelerate your body's natural anti-inflammatory and recovery responses. Treatment sites include back, knee, shoulder, ankle, wrist and elbow.
The OrthoCor Active System is indicated for adjunctive use in the palliative treatment of post-operative pain and edema in superficial soft tissue. The Active System temporarily relieves minor muscle and joint aches and pain associated with overexertion, strains, sprains, and arthritis. OrthoCor is the only wearable device on the market with patented Pulsed Electromagnetic Field (PEMF) therapy, one of the most advanced restorative therapies available today. PEMF works at the source of the injuries, and helps accelerate your body's natural anti-inflammatory and recovery responses. Treatment sites include back, knee, shoulder, ankle, wrist and elbow.- Drug-free pain relief
- Wearable design for therapy on the go
- Noninvasive and easy-to-use
- Helps return your patient to an active lifestyle
-


 The Recovery Shop provides patients direct access and convenience to the key products their physician recommends in assisting them in their recovery. These are products not covered by insurance.
The Recovery Shop provides patients direct access and convenience to the key products their physician recommends in assisting them in their recovery. These are products not covered by insurance.
- Unique E-commerce portal for each practice/physician
- Secure transaction functionality via CC/HSA
- Customized product offering per physician recommendation
- Direct delivery to the patient’s home
- No implementation costs or investment
- Ancillary revenue to the practice
- Decreased burden on staff
The Recovery Shop was born out of an idea that patients need somewhere to purchases products that are not covered by their insurance. Insurances are covering less and less of everything! These products used to be easy to get at your local mom and pop store/pharmacy. Those days are gone. People are directed to large online retailers where there are hundreds of options to choose from. This causes confusion for the patient and increases questions that have tobe answered by the doctorsstaff. PlusCVS and Amazon are making all ofthe money. We thought there has tobe a better way!
Who Is The Recovery Shop For?- ANY Individual Doctor, Physician Group, Hospital or Surgery Center
- Anyone referring patients to buy products off Amazon
- Anyone using a Joint Program Coordinator/Navigator
How It Works
- We create an E-Commerce website for each physician, group, hospital or surgery center
- Each individual doctor has the ability toselect products that they would prefer their patients to buy and use for post-op recovery
- Selections are made by body part and/or surgical procedure
- Patients only see what the physicians want them to see
- Products are Cash Pay/HSA Pay Only –We do not accept Insurance (these products are not covered by insurances)
Upon booking surgery, the patient is provided with an instruction sheet (via email or pre-op packet) which directs patients to the website.
- Patients select their group/hospital/center
- Patients select their physician
- Patients select their body part/surgical procedure
- Patients see “Products Recommended by your Physician”
- Patients choose which products they would like to purchase and put in their credit card (like any other e-commerce website)
- Patients receive a confirmation email and products are delivered to their house (via FedEx/UPS) in 1-5 business days
This is your webpage. We can Sell anything you think is appropriate to help patients with their recovery.
Some of the Products:- Cold Therapy Machines (NICE, BREG, OSSUR)
- Aids to Daily Living (ADLS)
- Bathroom Safety Items (Raised toilet seat, shower chair etc.)
- Scar Cream
- Recovery Items –Massage Gun, Foam Rollers
- Compression Socks
- DVT Units
- Nutrition Supplements
-

 The PlasmaFlow™ is intended to be an easy-to-use sequential compression system prescribed by a physician, for use in the home or clinical setting to help prevent the onset of DVT in patients by stimulating blood flow in the extremities by simulating muscle contractions. This device is used to aid in preventing DVT by enhancing blood circulation, diminishing post-operative pain and swelling, and enhancing wound healing. Features:
The PlasmaFlow™ is intended to be an easy-to-use sequential compression system prescribed by a physician, for use in the home or clinical setting to help prevent the onset of DVT in patients by stimulating blood flow in the extremities by simulating muscle contractions. This device is used to aid in preventing DVT by enhancing blood circulation, diminishing post-operative pain and swelling, and enhancing wound healing. Features:- Long-lasting rechargeable battery. Up to 10 hours of use on one charge
- 2 Modes: Continuous inflation and Step-Up inflation technology which allows the unit to increase pressure in slower increments to 55mmHG
- 2 LCD screens to monitor usage and pressure
- Portable, lightweight, and tubeless
-

 Squid operates by combining sequential compression with cold therapy. Each Squid wrap was uniquely developed for a specific body part with a sequential compression bladder* designed to direct swelling away from the treated body part while driving cold deep into the tissue. The combination of directional sequential compression and deep penetrating cold may help in reducing edema and pain while allowing better blood circulation in the treated area and reducing inflammation. For athletes Squid is an excellent recovery tool providing high efficacy cold therapy while driving metabolites such as lactic acid away from the treated muscle, increasing local circulation and getting you ready faster for more action! The sequential compression and cold work together to remove lactic acid and drive the cold deep into the treated muscle or joint. MUSCLE & JOINT RELIEF
Squid operates by combining sequential compression with cold therapy. Each Squid wrap was uniquely developed for a specific body part with a sequential compression bladder* designed to direct swelling away from the treated body part while driving cold deep into the tissue. The combination of directional sequential compression and deep penetrating cold may help in reducing edema and pain while allowing better blood circulation in the treated area and reducing inflammation. For athletes Squid is an excellent recovery tool providing high efficacy cold therapy while driving metabolites such as lactic acid away from the treated muscle, increasing local circulation and getting you ready faster for more action! The sequential compression and cold work together to remove lactic acid and drive the cold deep into the treated muscle or joint. MUSCLE & JOINT RELIEFSquid is designed to provide tremendous relief from muscle and joint pain.
This may allow users to reduce dependency on pain medication, perform everyday tasks that were previously painful, and enjoy walking, running, skiing, playing tennis, golf and other sports again. INJURY RECOVERYSquid revolutionizes injury recovery by combining sequential intermittent compression with cold treatment to provide maximum results.
Squid drives the cold treatment deep into the tissue, reducing swelling and directing fluids away from the treated area towards the heart. Swelling is reduced, thereby improving blood flow to the injured tissue and delivering much needed nutrients and oxygen. PERFORMANCE RECOVERYSquid's patent pending sequential intermittent compression assists many world-class athletes in post-workout and post-performance recovery.
Squid can be used in many ways: from elbow wraps for tendonitis, to shoulder wraps for pitchers, to leg wraps for runners and soccer players. Squid is designed to speed up performance recovery by helping remove lactic acid and other metabolites from muscles. -

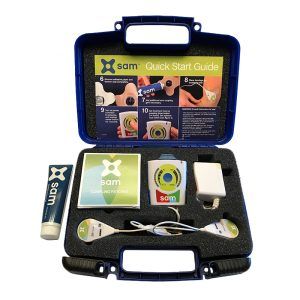 Developed and clinically proven to reduce chronic joint pain and accelerate soft tissue healing, sam® is the only FDA cleared long duration home use ultrasound device.
Developed and clinically proven to reduce chronic joint pain and accelerate soft tissue healing, sam® is the only FDA cleared long duration home use ultrasound device.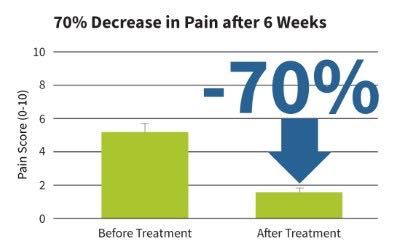
- The sam® wearable long duration continuous ultrasound device was developed by millions of dollars of government healthcare funding to reduce surgery and treat chronic pain. i. Link: https://samrecover.com/clinical-evidence/ ii. Link: https://www.nimhd.nih.gov/news-events/features/clinical-health-services/wearable-ultrasound.html
- The wearable ultrasonic soft-tissue healing and pain management device is recommended and used by tens of thousands of elite athletes, injured workers and military personnel i. Link: https://samrecover.com/testimonials/
Lewis Jr GK, Langer MD, Henderson Jr CR, Ortiz R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound in medicine & biology. 2013 Aug;39(8):1429. https://www.ncbi.nlm.nih.gov/pubmed/23743101
Petterson S, Plancher K, Klyve D, Draper D, Ortiz R. Low-Intensity Continuous Ultrasound for the symptomatic treatment of upper should and neck pain: A randomized, double-blind placebo-controlled clinical trial. Journal of Pain Research, Accepted 2020.
Ilter L, Dilek B, Batmaz I, Ulu MA, Sariyildiz MA, Nas K, Cevik R. Efficacy of pulsed and continuous therapeutic ultrasound in myofascial pain syndrome: a randomized controlled study. American journal of physical medicine & rehabilitation. 2015 Jul 1;94(7):547-54.
https://www.ncbi.nlm.nih.gov/pubmed/25299534
Yildirim MA, Öneş K, Gökşenoğlu G. Effectiveness of Ultrasound Therapy on Myofascial Pain Syndrome of the Upper Trapezius: Randomized, Single-Blind, Placebo-Controlled Study. Arch Rheumatol. 2018 Mar 23;33(4):418-423. doi: 10.5606/ArchRheumatol.2018.6538. PMID: 30874250; PMCID: PMC6409164
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6409164/
Xia P, Wang X, Lin Q, Cheng K, Li X. Effectiveness of ultrasound therapy for myofascial pain syndrome: a systematic review and meta-analysis. J Pain Res. 2017 Mar 7;10:545-555. doi: 10.2147/JPR.S131482. PMID: 28331357; PMCID: PMC5349701.
-
 The OrthoCor Active System is indicated for adjunctive use in the palliative treatment of post-operative pain and edema in superficial soft tissue. The Active System temporarily relieves minor muscle and joint aches and pain associated with overexertion, strains, sprains, and arthritis. OrthoCor is the only wearable device on the market with patented Pulsed Electromagnetic Field (PEMF) therapy, one of the most advanced restorative therapies available today. PEMF works at the source of the injuries, and helps accelerate your body's natural anti-inflammatory and recovery responses. Treatment sites include back, knee, shoulder, ankle, wrist and elbow.
The OrthoCor Active System is indicated for adjunctive use in the palliative treatment of post-operative pain and edema in superficial soft tissue. The Active System temporarily relieves minor muscle and joint aches and pain associated with overexertion, strains, sprains, and arthritis. OrthoCor is the only wearable device on the market with patented Pulsed Electromagnetic Field (PEMF) therapy, one of the most advanced restorative therapies available today. PEMF works at the source of the injuries, and helps accelerate your body's natural anti-inflammatory and recovery responses. Treatment sites include back, knee, shoulder, ankle, wrist and elbow.- Drug-free pain relief
- Wearable design for therapy on the go
- Noninvasive and easy-to-use
- Helps return your patient to an active lifestyle
-


Prescription-Strength Topical Pain Relief Patch
The Only Patch That Combines Lidocaine (Anesthetic) and Menthol (Analgesic) with Methyl Salicylate (NSAID) to Provide Long-Lasting Pain Relief. Medical Grade Adhesive Included.
LidoPro® Patch – Topical Pain Relief Patch & Medical Adhesive
A safe and effective pain relief patch to help treat and manage your pain. LidoPro® Patch combines Lidocaine (anesthetic) and Menthol (analgesic) with Methyl Salicylate (NSAID) to provide long lasting relief for both acute and chronic pain symptoms. Includes medical grade adhesive.
Designed to relieve muscle pain, joint pain, and injuries associated with backache, neckache, soreness, sprains, stiffness, or strains.
Active Ingredients Purpose
Lidocaine 4% - Topical Anesthetic
Menthol 5% - Topical Analgesic
Methyl Salicylate 4% - Nonsteroidal Anti-Inflammatory Drug (NSAID)
Inactive Ingredients:
Acrylic Acid, Aluminum Hydroxide, Carmellose Sodium, 2-Ethylhexyl Acrylate, Glycerin, Isopropyl Myristate, Methyl Acrylate, Nonoxynol-30, Polyacrylic Acid, Polysorbate 80, Sodium Polyacrylate, Sorbitan Sesquioleate, Starch, Talc, Tartaric Acid, Titanium Dioxide, Water
Directions
Adults 18 Years and Older:
- Clean and dry the affected area
- Open pouch and remove one patch containing medical adhesive backing
- Remove protective film from both patch and medical adhesive
- Apply 1 patch directly to the affected area of pain and leave in place for 8-12 hrs
- Only use one patch at a time
- Do not use more than 2 patches per day
- Wash hands with soap and water after applying or removing patch
- Reseal pouch containing unused patches after each use
- If allergic to any NSAIDS
- Any patch from a pouch that has been opened for 7 or more days
- On damaged or irritated skin
- With a bandage or heating pad
- Other than as directed
- Wash hands after applying or removing patch
- Avoid contact with eyes
Use Only As Directed by Your Physician
-
 The G-Force Back Brace provides soothing, cool comfort for sprains and strains of the lower spine, spinal stenosis, displacement of intervertebral discs, and chronic lower pain. At one time or another, lower back pain impacts 80% of Americans. This revolutionary brace affordably soothes the back while providing support for the lower lumbar area, allowing for faster recovery and increased levels of comfort. How Patients Benefit
The G-Force Back Brace provides soothing, cool comfort for sprains and strains of the lower spine, spinal stenosis, displacement of intervertebral discs, and chronic lower pain. At one time or another, lower back pain impacts 80% of Americans. This revolutionary brace affordably soothes the back while providing support for the lower lumbar area, allowing for faster recovery and increased levels of comfort. How Patients Benefit- Easy application and removal
- Removable gel pack
- Soothing comfort to aid in recovery from sprains and strains
- Decreased pain and swelling
- Snug fit to ensure targeted pain relief
- One size fits most
-

 The Circul8 Portable DVT is a sequential compression system, prescribed by a physician, for use in the home or clinical setting to help prevent the onset of DVT by stimulating blood flow in the extremities. How Patients Benefit
The Circul8 Portable DVT is a sequential compression system, prescribed by a physician, for use in the home or clinical setting to help prevent the onset of DVT by stimulating blood flow in the extremities. How Patients Benefit- Aid in the prevention of DVT
- Enhance blood circulation
- Diminish post-operative pain and swelling
- Reduce wound healing time
- Aid in the treatment and healing of: stasis dermatitis, venous stasis ulcers, arterial and diabetic leg ulcers, chronic venous insufficiency and reduction of edema in the lower limbs
-


SOOTHING TOPICAL PAIN RELIEF PATCH
Three Active Ingredients Combine To Not Only Fight Pain, But Also Attack Inflammation. VIVA Patch Features An Anti-Inflammatory – Providing You Hours of Relief.
VIVA Patch is a topical pain relief patch with Lidocaine, Camphor, and Methyl Salicylate (NSAID) that provides soothing pain and inflammation relief. Formulated without an overbearing scent.
Medical grade adhesive is included with each 15-count unit box.
Active Ingredients: Lidocaine 2.5%, Methyl Salicylate 4%, Camphor 2% Precautions- Patients should use no more than two patches per day
- Dispose of any patch from a pouch that has been opened for 7 or more days
- Safely discard used patches by folding the adhesive together.
- Discard away from small children and pets
- Clean and dry the affected area
- Open pouch and remove one patch
- Remove protective film from the medicated patch
- Carefully apply patch to the affected area of pain and leave in place for up to 12 hours
- If pain lasts after using the first patch, a second patch may be applied for up to another 12 hours
- Only use one patch at a time
- Wash hands after applying or removing patch
- Reseal pouch containing unused patches after each use
- Use Only As Directed by Your Physician
-


Prescription-Strength Pain Relief Available Over-The-Counter
The Only Formulation of Its Kind – An Anesthetic, Anti-Inflammatory, Analgesic, and Counterirritant Combine with Two Skin Conditioners
to Provide Patients with Powerful Relief from Pain and Inflammation.
LidoPro® Ointment – Topical Pain Relief Ointment
A safe and effective pain relief ointment to help treat and manage your pain. LidoPro® contains 4 active ingredients that provide anti-inflammatory (NSAID), analgesic, and anesthetic properties to address both acute and chronic pain symptoms. Built with a hands-free applicator for easy application.
Designed to relieve muscle pain, joint pain, and injuries associated with backache, neckache, soreness, sprains, stiffness, or strains.
Active Ingredients Purpose
Lidocaine 4% - Topical Anesthetic
Menthol 10% - Topical Analgesic
Capsaicin 0.0325% - Counterirritant
Methyl Salicylate 27.5% - Nonsteroidal Anti-Inflammatory Drug (NSAID)
Inactive Ingredients:
Allantoin, Aloe Barbadensis Leaf Juice, Ammonium Acryloyldimethyltaurate/VP Copolymer, Cetyl Alcohol, Chamomilla Recutita Matricaria Flower Extract, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Inulin Lauryl Carbamate, PEG-100 Stearate, Phenoxyethanol, Stearic Acid, Triethanolamine, Water.
Directions
Adults 18 Years and Older:
- Clean and dry the affected area
- Apply product directly to your skin, up to 4 times daily
- Wash hands after use with warm, soapy water for 30-45 seconds
Other Information
- Store in a cool, dry place with lid tightly closed
- Do Not Use:
- If allergic to any NSAIDS
- On damaged or irritated skin
- With a bandage or heating pad
- Other than as directed
- Condition worsens
- Excessive skin irritation develops
- Symptoms persist for more than 7 days, or symptoms clear up and occur again within 3 days

