Redefine Cervical Fusion Recovery
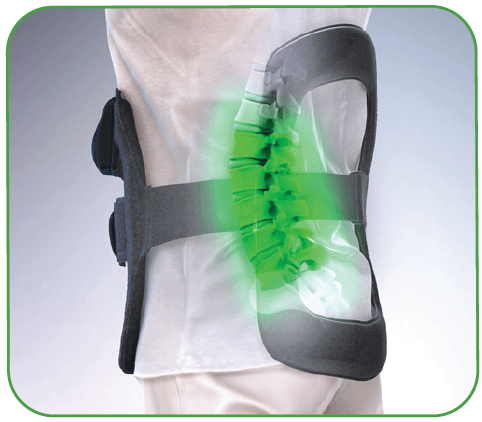
The CervicalStim™ device provides a safe and effective non-surgical treatment to improve fusion success rates. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing.

The only bone growth stimulation therapy device approved by the FDA as a noninvasive adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion.
Proven Effective Therapy
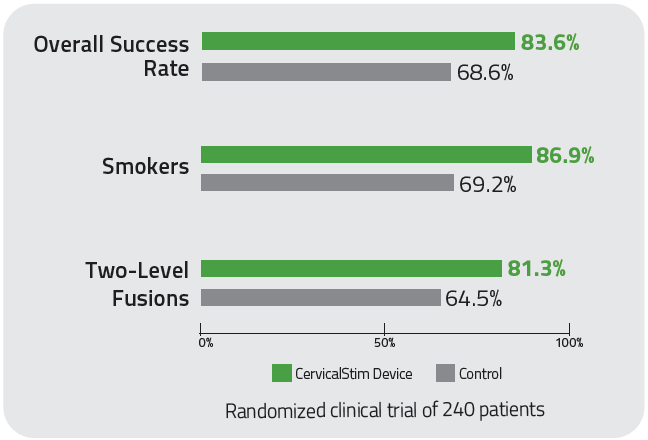
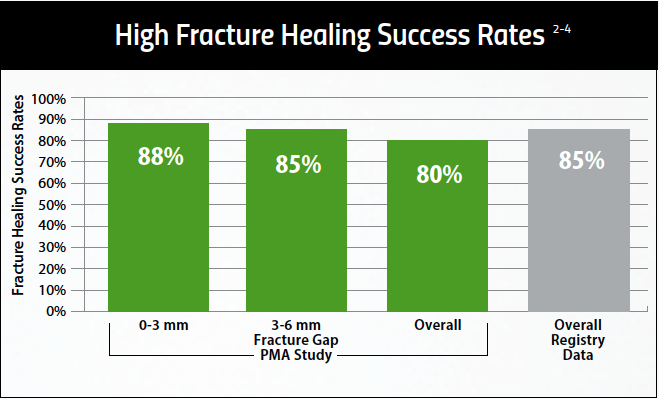
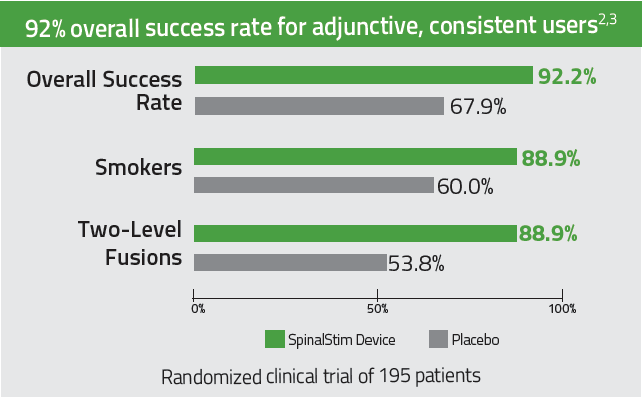
With an 84% overall clinical success rate, the CervicalStim device provides2,3
- A 22% increase in cervical fusion success when used adjunctively to surgery2,3
- Statistically significant results for smokers and multi-level fusion patients2,3
- 360 degrees of PEMF treatment around the fusion site4
- Coverage up to 5 vertebral levels of fusion4
Brief Prescribing Information:
The CervicalStim™ device is indicated as an adjunct to cervical fusion surgery in patients at high risk for non-fusion; there are no known contraindications.
Do not use this device if you have a cardiac pacemaker or defibrillator. Remove the device prior to any imaging procedures. Adverse effects may include increased pain, numbness and tingling, headache, migraines and nausea; these effects may or may not be directly related to use of the device.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Services at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
References: 1. iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP19_RPT), iData Research Inc (www.idataresearch.net) 2019. 2. PMA P030034. December 2004. 3. Foley KT, Mroz TE, Arnold PM, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8(3):436-442. 4. Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic.