-
 All 5 spine braces have patented Cryogel packs inside designed to provide soothing, cool comfort for sprains and strains of the lower spine, chronic low back pain, and back pain resulting from surgery or injury. The addition of moveable, rigid side panels provide lateral support and a comfortable environment for healing. One-size adjustable, the CryoBack Braces comfortably fits waists ranging from 24-70 inches.
All 5 spine braces have patented Cryogel packs inside designed to provide soothing, cool comfort for sprains and strains of the lower spine, chronic low back pain, and back pain resulting from surgery or injury. The addition of moveable, rigid side panels provide lateral support and a comfortable environment for healing. One-size adjustable, the CryoBack Braces comfortably fits waists ranging from 24-70 inches. -

The Stars Sleeper Brace is a patented post-operative shoulder brace designed for shoulder and arm immobilization following both arthroscopic and open shoulder surgeries. Its patented design allows for comfort and stability during both ambulation and sleep.
During ambulation, the arm rests comfortably in the cut-out foam cradle; during sleep, the posterior cradle elevates the arm while moving it forward, relieving pressure on the operative site.
-

The Boxers Splint™ is a patented fracture brace designed for the treatment of acute and sub-acute fractures of the 4th and 5th metacarpal shaft and neck commonly known as the Boxers Fracture. The Boxers Splint™ comes in four sizes S, M, L, XL. It also comes in Standard and Extended length sizes.
The Boxers Splint™ is the only off the shelf product that can be customized with a hair dryer by heating the shell and molding it to the fracture to afford better conformability and reimbursement.
-
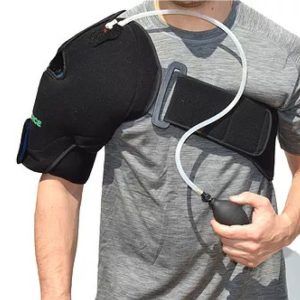
The Eclipse CryoShoulder from G-Force Braces helps patients overcome shoulder pain and swelling, whether it is chronic shoulder pain, post-surgical swelling, or post-exercise swelling and soreness. It combines targeted compression with cold, providing more rapid recovery.
The Eclipse includes two reusable gel packs and comes in one universal size.
-

The Orbit ROM CryoKnee brace is designed to combine the benefits of pneumatic compression with cold therapy to diminish swelling and decrease pain. Whether you’re an athlete looking for a quicker recovery or a post-operative knee surgery patient, you will enjoy a more comfortable recovery with the G-Force Knee ColdKompressor.
The Orbit includes two reusable gel packs and comes in one universal size.
-
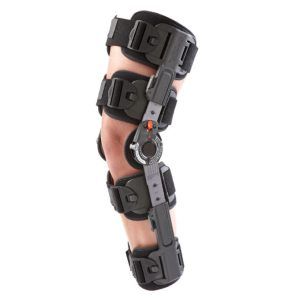
The Galaxy T-Scope ROM Hinged Knee brace is designed to provide protected, controlled range-of-motion (ROM) for patients recovering from knee surgery or those who have knee injuries or instabilities.
Telescoping calf and thigh sleeves for sizing a wide range of patients from 5′ to 6’4″ tall
Accommodates both right and left leg procedures.
-

 HIGH ENERGY PRESSURE WAVE THERAPY FROM THE INVENTORS OF SWISS RADIAL SHOCK WAVE High energy radial pressure waves to relieve musculoskeletal pain quickly. CLINICALLY PROVEN HIGH ENERGY OUTPUT DolorClast® Blue handpiece delivers constant high energy density and output pressure at all frequencies Up to 25 hertz frequency enabling 1500 high energy pulses per minute for fast treatments Special treatment modes to achieve best possible patient comfort 7 applicators with fast twist release for quick applicator changing Fast return on investment for your practice YOUR HOLISTIC SOLUTION FOR MUSCULOSKELETAL PAIN THERAPY
HIGH ENERGY PRESSURE WAVE THERAPY FROM THE INVENTORS OF SWISS RADIAL SHOCK WAVE High energy radial pressure waves to relieve musculoskeletal pain quickly. CLINICALLY PROVEN HIGH ENERGY OUTPUT DolorClast® Blue handpiece delivers constant high energy density and output pressure at all frequencies Up to 25 hertz frequency enabling 1500 high energy pulses per minute for fast treatments Special treatment modes to achieve best possible patient comfort 7 applicators with fast twist release for quick applicator changing Fast return on investment for your practice YOUR HOLISTIC SOLUTION FOR MUSCULOSKELETAL PAIN THERAPY Please contact us to learn more.
Please contact us to learn more. -


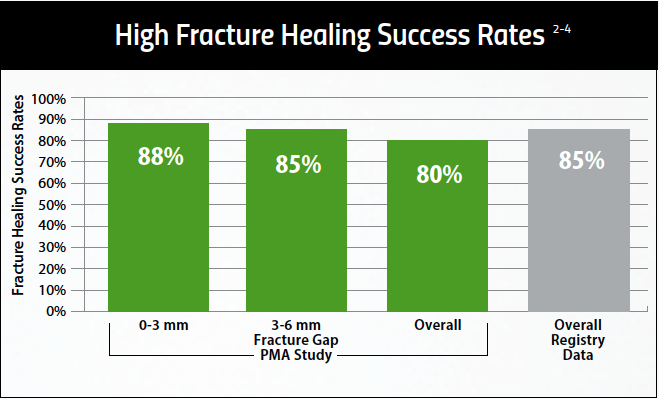
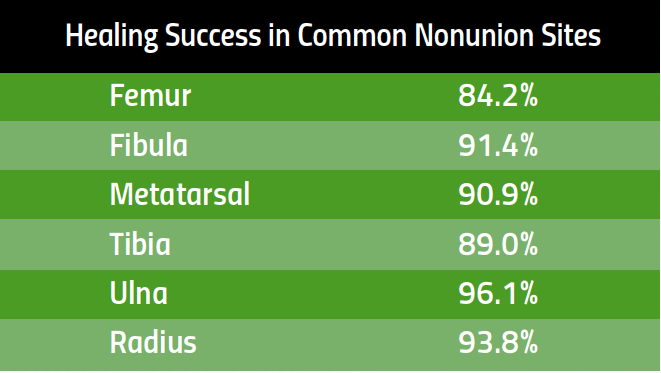
Healing Success in Common Nonunion Fractures
PhysioStim™ devices provide a safe and effective non-surgical treatment to improve nonunion fracture healing. These devices use a pulsed electromagnetic field (PEMF) signal to induce a low-level electrical field at the fracture site which stimulates bone healing. 2,3
Proven Effective Therapy
Anatomically Designed Models

- 360 degrees of PEMF treatment around the fracture site that evenly penetrates across tissue, bone and fixation1,7
- Single-piece, cordless design that allows for ease of placement and patient mobility
Commitment to Outcomes
PhysioStim devices are accompanied by the STIM onTrack™ mobile app, which provides patients with a treatment calendar, therapy reminder, outcome measurement tools and additional educational resources.
STIM onTrack mobile app combined with the Orthofix DIRECT™ Physician Portal enables physicians to remotely:
- View patient adherence to prescribed treatments
- View patient reported outcome measures (PROM) questionnaire responses
- Manage new and submitted patient prescriptions
Brief Prescribing Information:
The PhysioStim™ device is indicated for the treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and all flat bones, where the width of the nonunion defect is less than one-half the width of the bone to be treated. A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Use of this device is contraindicated where the individual has synovial pseudarthrosis. Demand type pacemaker operation may be adversely affected by exposure to pulsed electromagnetic fields. The safety and effectiveness of this device has not been established for individuals lacking skeletal maturity or individuals with a nonunion secondary to, or in connection with, a pathological condition. The safety of this device for use on patients who are pregnant or nursing has not been established. Rare instances of reversible minor discomfort have been reported.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Services at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
References: 1. Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic 2. PMA P850007. February 1986 3. Garland DE, Moses B, Salver W. Fracture healing: Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 1991;22(3):295-302 4. Orthofix patient registry. PMA P850007/S20. Data on file. 5. iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP19_RPT), iData Research Inc (www.idataresearch.net) 2017 6. iData Research Inc., U.S. Market for Orthopedic Trauma Devices (iDATA_USTRA19_RMS), iData Research Inc (www.idataresearch.net) 2019 7. Navarro, M., Michiardi, A., Castano, O., & Planell, J.. (2008). Biomaterials in orthopaedics. Journal of the Royal Society Interface, 5(27), 1137-1158

-


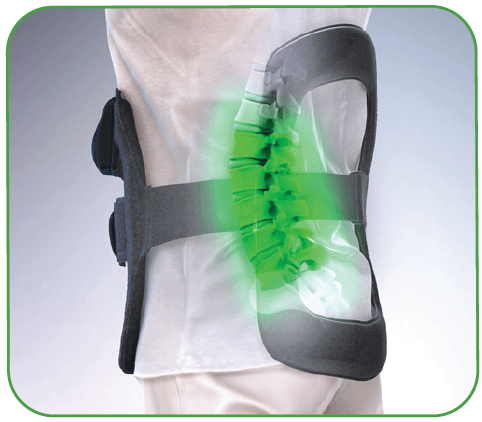
Redefine Cervical Fusion Recovery
The CervicalStim™ device provides a safe and effective non-surgical treatment to improve fusion success rates. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing.

The only bone growth stimulation therapy device approved by the FDA as a noninvasive adjunctive treatment option for cervical fusion in patients at high-risk for non-fusion.
Proven Effective Therapy
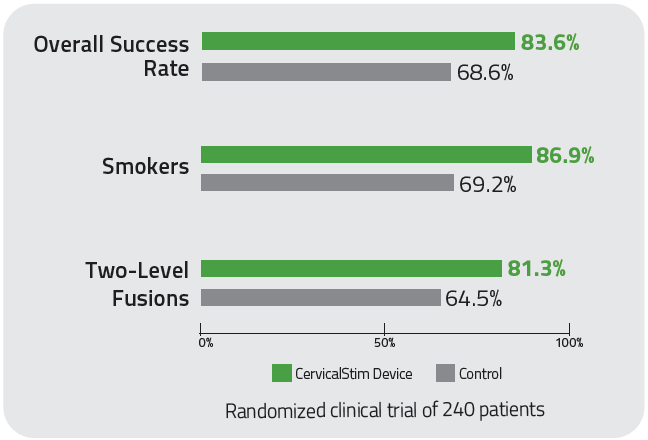
With an 84% overall clinical success rate, the CervicalStim device provides2,3
- A 22% increase in cervical fusion success when used adjunctively to surgery2,3
- Statistically significant results for smokers and multi-level fusion patients2,3
- 360 degrees of PEMF treatment around the fusion site4
- Coverage up to 5 vertebral levels of fusion4
Brief Prescribing Information:
The CervicalStim™ device is indicated as an adjunct to cervical fusion surgery in patients at high risk for non-fusion; there are no known contraindications.
Do not use this device if you have a cardiac pacemaker or defibrillator. Remove the device prior to any imaging procedures. Adverse effects may include increased pain, numbness and tingling, headache, migraines and nausea; these effects may or may not be directly related to use of the device.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Services at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
References: 1. iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP19_RPT), iData Research Inc (www.idataresearch.net) 2019. 2. PMA P030034. December 2004. 3. Foley KT, Mroz TE, Arnold PM, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8(3):436-442. 4. Data on file. Field mapping analysis conducted by M. Zborowski, Ph.D., Cleveland Clinic.

-


Increase Success Rates in Spinal Fusion
The SpinalStim™ device provides a safe and effective non-surgical treatment to improve fusion healing. The device uses a pulsed electromagnetic field (PEMF) to induce a low-level electrical field at the fusion site which stimulates bone healing.2-4
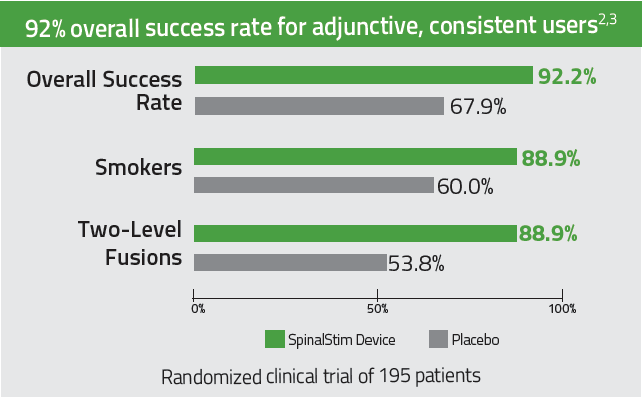
The only bone growth stimulation therapy device approved by the FDA as both a lumbar spinal fusion adjunct and as a non-surgical treatment for spinal pseudarthrosis.2-4
92% overall clinical success rate for adjunctive, consistent users. 2-3
Improves success rates in spinal fusions by 36% 2,3
- 360 degrees of pulsed electromagnetic field (PEMF) treatment around the fusion site that evenly penetrates across tissue, bone and fixation5,6
- PEMF coverage up to 5 vertebral levels5
- NASS coverage recommendations support the use of PEMF stimulation as an adjunct to spinal fusion surgery in high-risk patients7
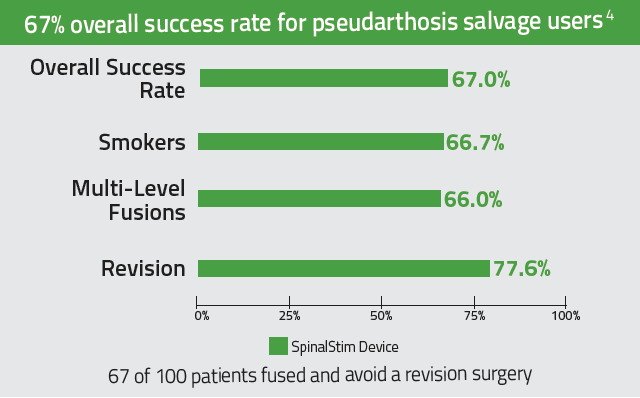
- Adjunctive PEMF treatment can be recommended for patients who are at high risk for pseudarthrosis8
Brief Prescribing Information:
The SpinalStim™ device is indicated as a spinal fusion adjunct to increase the probability of fusion success and as a nonoperative treatment of salvage of failed spinal fusion, where a minimum of nine months has elapsed since the last surgery. Cardiac pacemakers may be adversely affected by exposure to pulsed electromagnetic fields. Use of this device is contraindicated where the individual has an implanted cardiac pacemaker.
Full prescribing information can be found in product labeling on our patient education website BoneGrowthTherapy.com or by calling Patient Services at 1-800-535-4492.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
References: 1. iData Research Inc., U.S. Market for Spinal Implants and VCF (iDATA_USSP19_RPT), iData Research Inc (www.idataresearch.net) 2017. 2. PMA P850007/S6. February 1990. 3. Mooney V. Pulsed electromagnetic fields: an adjunct to interbody spinal fusion surgery in the high risk patient. Surg Technol Int 1993, 2:405-410. 4. Simmons JW Jr, Mooney V, Thacker I. Pseudarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electro-magnetic fields. Am J Orthop. 2004;33(1):27-30. 5. Zborowski M, Androjna C, Waldorff EI, Midura RJ. Comparison of therapeutic magnetic stimulation with electric stimulation of spinal column vertebrae. IEEE Transactions on Magnetics, Vol. 51, No. 12, December 2105, 5001009. Erratum in IEEE Transactions on Magnetics, Vol. 53, No. 2, February 2017, 9700101. 6. Navarro, M., Michiardi, A., Castano, O., & Planell, J.. (2008). Biomaterials in ortho-paedics. Journal of the Royal Society Interface, 5(27), 1137-1158. 7. Spine.org. 8. Ethan Cottrill, MS, Zach Pennington, BS, A. Karim Ahmed, BS, Daniel Lubelski, MD, Matthew L. Goodwin, MD, PhD, Alexander Perdomo-Pantoja, MD, Erick M. Westbroek, MD, Nicholas Theodore, MD, Timothy Witham, MD, and Daniel Sciubba, MD Department of Neurosurgery, The Johns Hopkins School of Medicine, Baltimore, Maryland The effect of electrical stimulation therapies on spinal fusion: a cross-disciplinary systematic review and meta-analysis of the preclinical and clinical data SOURCE: J Neurosurg Spine, October 8, 2019.

-


The NICE1 uses advanced technology to greatly improve the convenience and efficacy of cold + compression therapy without ice.
It also provides programmable pneumatic compression which is proven to reduce edema and speed recovery. NICE1 integrates these highly effective therapies in a small (8 x 8 x 8 inches) and lightweight (9 lbs.) package with an easy-to-use touch screen interface.- NO ICE - NICE1 is an iceless system. This is a clear point of differentiation when compared with other cold therapy devices.
- SMALLER & LIGHTER - NICE1 is the smallest and lightest cold + compression therapy device on the market.
- SIMPLICITY - NICE1 has an extremely intuitive graphical touch screen interface that makes it easy to operate.
- DESIGN-FORWARD - NICE1 is focused on a superior user experience, blending pro-tested design with state-of-the-art technology.
-
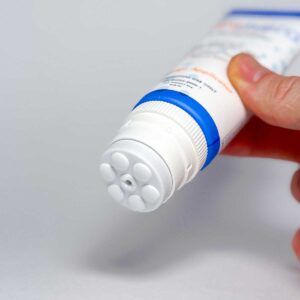

Lidocaine 4%, Cinnamomum, Arnica, Boswellia, Aloe, Turmeric, Eucalyptus Oil with Mess-free Massage Applicator Cap
- OTC Pain Relief – Cinthera is a non-compounded advanced strength OTC medication. Distributed only through healthcare provider networks.
- Trusted Ingredients – An effective combination of active & inactive ingredients to soothe pain and protect skin.
- Targeted Application – Massage Applicator Cap delivers medication exactly where you need it - without the mess.
- Registered with FDA – Compliant with the FDA M017 OTC Monograph and listed with all major pricing databases.

