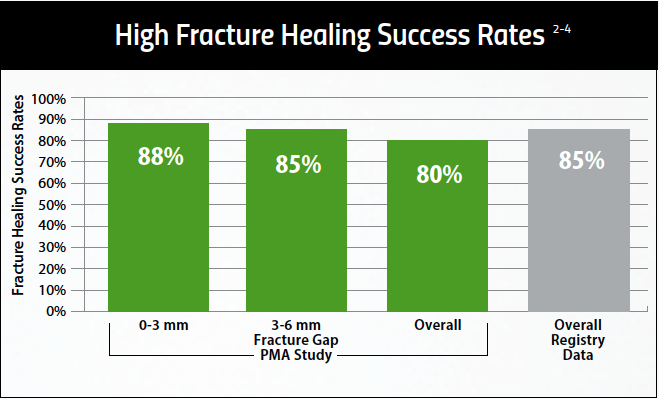
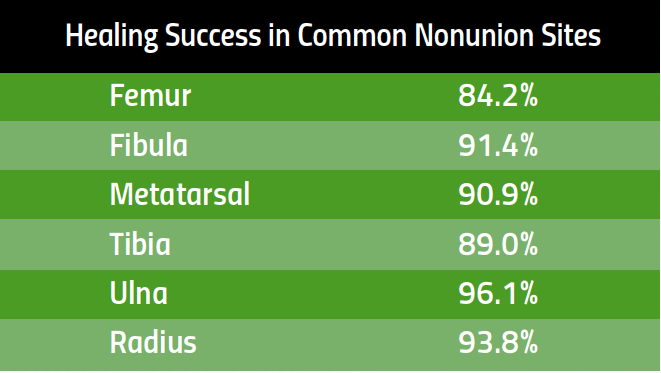
Developed and clinically proven to reduce chronic joint pain and accelerate soft tissue healing, sam® is the only FDA cleared long duration home use ultrasound device.
 Joint Arthritis Pain Treatment with sam®:
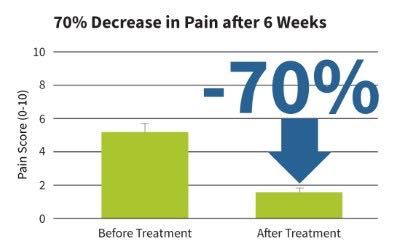
Joint Arthritis Pain Treatment with sam®: In a clinical research study on sam® long duration continuous ultrasound treatment for knee osteoarthritis, patients experienced a 52% pain reduction and 20% improvement in joint function, which were statistically significant (p<0.05). (Langer, 2014). In another placebo-controlled study on arthritis pain, sam® long duration continuous ultrasound treatment reduced joint pain by 2.5 points (48%) over six weeks of treatment which was statistically significant over the placebo 1.23 point decrease (p<0.03). (Langer, 2015). In a third double-blind placebo controlled clinical study on sam® for joint arthritis pain, patients had significant 1.96 point (40%) pain reduction for active (p<0.0001) vs. 0.85 point reduction for placebo treatment (p<0.13). Active sam® treatment patients also had significant improvement in pain, stiffness and function on the Western Ontario and McMaster Universities (WOMAC) scale compared with placebo (500 vs. 311 respectively, p<0.02). Rotational strength of the treated knee was increased by 3.2N, p=0.03) in the active treatment group. (Draper, 2018). A recent 2019 systematic review and meta-analysis on therapeutic ultrasound diathermy for knee osteoarthritis included 15 studies. The results demonstrated that therapeutic ultrasound significantly relieved pain (p<0.00001) and reduced the Western Ontario and McMaster Universities (WOMAC) physical function score (p=0.03). In addition, therapeutic ultrasound increased the active range of motion (p<0.00001) and reduced the Lequesne index (p<0.00001). The authors concluded that therapeutic ultrasound is a safe treatment to relieve pain and improve physical function in patients with knee osteoarthritis. (Wu, 2019).
Langer MD, Levine V, Taggart R, Lewis GK, Hernandez L, Ortiz R. Pilot Clinical Studies of Long Duration, Low Intensity Therapeutic Ultrasound for Osteoarthritis. Proc IEEE Annu Northeast Bioeng Conf.
2014 Apr;2014:14789673. doi: 10.1109/NEBEC.2014.6972850. PMID: 25788823; PMCID: PMC4361017.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4361017/
Langer MD, Lewis GK Jr. Sustained Acoustic Medicine: A Novel Long Duration Approach to Biomodulation Utilizing Low Intensity Therapeutic Ultrasound. Proc SPIE Int Soc Opt Eng.
2015 May;9467:94670I. doi: 10.1117/12.2178213. PMID: 30078928; PMCID: PMC6070146.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6070146/
Draper DO, Klyve D, Ortiz R, Best TM. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: a randomized, placebo-controlled double-blind study. J Orthop Surg Res.
2018 Oct 16;13(1):257. doi: 10.1186/s13018-018-0965-0. PMID: 30326947; PMCID: PMC6192104.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6192104/
Wu Y, Zhu S, Lv Z, Kan S, Wu Q, Song W, Ning G, Feng S. Effects of therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis. Clinical rehabilitation.
2019 Dec;33(12):1863-75.
https://www.ncbi.nlm.nih.gov/pubmed/31382781
Upper Back, Neck and Shoulder Pain Treatment with sam®: A double-blind placebo controlled clinical trial of a self-applied sam® long duration ultrasound device was compared to placebo for trapezius myofascial pain over 10 treatment sessions. Active sam® treatment demonstrated a significant 190% reduction in pain and 158% improvement in health compared to placebo after only 1-hour of treatment (p<0.05) (Lewis, 2013). An additional double-blind placebo controlled study on sam® for trapezius myofascial pain was completed. Patients treated with active therapy observed a significant mean pain reduction from baseline of 2.61-points for active (p<0.001), compared to 1.58-points reduction from baseline for placebo (p=0.087), resulting in a significant 1.03-point greater decrease in active over placebo (p=0.003). Overall health quality score was significantly higher in the active group at 2.84 points compared to the placebo group at 0.46 points (p<0.001). (Patterson, 2020). Two recent 60 and 54 subject placebo-controlled studies on therapeutic ultrasound diathermy for trapezius myofascial pain demonstrated significant reduction in pain for active continuous ultrasound, respectively. The authors concluded that therapeutic ultrasound is an effective treatment of myofascial pain syndrome, and continuous therapy is the most effective. (Ilter, 2015), (Yildirim, 2018). A systematic review and meta-analysis (1966-2016) on therapeutic ultrasound including 10 studies and 428 subjects demonstrated that therapeutic ultrasound significantly reduced pain intensity and increased pain threshold. (Xia, 2017).
Lewis Jr GK, Langer MD, Henderson Jr CR, Ortiz R. Design and evaluation of a wearable self-applied therapeutic ultrasound device for chronic myofascial pain. Ultrasound in medicine & biology. 2013 Aug;39(8):1429.
https://www.ncbi.nlm.nih.gov/pubmed/23743101
Petterson S, Plancher K, Klyve D, Draper D, Ortiz R. Low-Intensity Continuous Ultrasound for the symptomatic treatment of upper should and neck pain: A randomized, double-blind placebo-controlled clinical trial. Journal of Pain Research, Accepted 2020.
Ilter L, Dilek B, Batmaz I, Ulu MA, Sariyildiz MA, Nas K, Cevik R. Efficacy of pulsed and continuous therapeutic ultrasound in myofascial pain syndrome: a randomized controlled study. American journal of physical medicine & rehabilitation. 2015 Jul 1;94(7):547-54.
https://www.ncbi.nlm.nih.gov/pubmed/25299534
Yildirim MA, Öneş K, Gökşenoğlu G. Effectiveness of Ultrasound Therapy on Myofascial Pain Syndrome of the Upper Trapezius: Randomized, Single-Blind, Placebo-Controlled Study. Arch Rheumatol. 2018 Mar 23;33(4):418-423. doi: 10.5606/ArchRheumatol.2018.6538. PMID: 30874250; PMCID: PMC6409164
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6409164/
Xia P, Wang X, Lin Q, Cheng K, Li X. Effectiveness of ultrasound therapy for myofascial pain syndrome: a systematic review and meta-analysis. J Pain Res. 2017 Mar 7;10:545-555. doi: 10.2147/JPR.S131482. PMID: 28331357; PMCID: PMC5349701.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5349701/






 The Recovery Shop provides patients direct access and convenience to the key products their physician recommends in assisting them in their recovery. These are products not covered by insurance.
The Recovery Shop provides patients direct access and convenience to the key products their physician recommends in assisting them in their recovery. These are products not covered by insurance.